Food safety and hygiene newsletter Sept. 2025

This is an electronic newsletter updating regulations and laws on food hygiene and safety in domestic and international markets, Sept. 2025 issue.
Australia’s Newly Revised MRLs in 09/2025: Australia Amends MRL Standard
On Septemrber 2, 2025, Food Standards Australia New Zealand (FSANZ) published Amendments No. APVMA 2, No. APVMA 3 and No. APVMA 4, 2025 to vary Schedule 20 of the Food Standards Code— Schedule 20 Maximum residue limits. The amendment takes effect immediately.
- MRLs in food have been revised for the following substances: Boscalid, Pyraclostrobin, Pyroxasulfone, Spinetoram, Chlorantraniliprole, Cyantraniliprole, Metalaxyl.
- MRLs in food have been newly established for the following substance: Aminoethoxyvinylglycine, Famoxadone, MCPA, Methoxyfenozide, Fluvalinate, Imazamox, Dithiocarbamates, Cyclobutrifluram, Bifenthrin, Chlorantraniliprole, Isoxaben, Metalaxyl, Propamocarb, Trichlorfon.
For example cases change MRLs as below:
|
Active Pesticides |
Food |
Before (ppm) |
After (ppm) |
|
Aminoethoxyvinylglycine |
Avocado |
- |
0.05 |
|
Boscalid |
Blueberries |
15 |
10 |
|
Famoxadone |
Meat (mammalian) (in the fat) ; Milks |
- |
0.01 |
|
Edible offal (mammalian); |
- |
0.05 |
|
|
Poultry meat (in the fat); Poultry, edible offal of; Eggs |
- |
0.01 |
|
|
Leafy vegetables |
- |
40 |
|
|
MCPA |
Sugar cane |
- |
0.01 |
|
Methoxyfenozide |
Carob |
- |
5 |
|
Pyraclostrobin |
Blueberries |
5 |
4 |
|
Dithiocarbamates |
Swede |
- |
1 |
|
Turnip, garden |
- |
1 |
|
|
Fluvalinate |
Macadamia nuts |
- |
0.01 |
|
Imazamox |
Maize cereals (subgroup) |
- |
0.02 |
|
Pyroxasulfone |
Cereal grains (except maize; popcorn and sweet corns) |
0.01 |
0.02 |
|
Spinetoram |
Cacao beans |
0.01 |
0.05 |
|
Cyclobutrifluram |
Barley, Wheat |
- |
0.01 |
|
Poultry meat; Poultry, edible offal of; Eggs |
- |
0.03 |
|
|
Edible offal (mammalian) |
- |
0.5 |
|
|
Meat (mammalian); Milks |
- |
0.05 |
|
|
All other foods except animal food commodities |
- |
0.05 |
|
|
Bifenthrin |
Pecan |
- |
0.05 |
|
Chlorantraniliprole |
Rice bran, unprocessed |
- |
5 |
|
Rice |
0.4 |
3 |
|
|
Cyantraniliprole |
Avocado |
1 |
0.5 |
|
Isoxaben |
Pulses |
- |
0.01 |
|
Metalaxyl |
Peach |
0.2 |
0.6 |
|
Almonds |
5 |
2 |
|
|
Propamocarb |
Ginger |
- |
50 |
|
Trichlorfon |
Leafy vegetables |
- |
15 |
For details see attached link: https://www.legislation.gov.au/F2015L00468/latest/versions
US
Changes pesticide residue limits in or on certain products in 08/2025
In August 2025, the US Code of Federal Regulations (CFR) did not have many changes, specifically on August 15, 2025, only the maximum limit for the pesticide: Mandipropamide in Papaya was added at 0.9 ppm.
For details see attached link: https://www.ecfr.gov/compare/2025-08-15/to/2025-08-14/title-40/chapter-I/subchapter-E/part-180/subpart-C/section-180.637
Proposal to remove the color additive listing for use of Orange B on casings or surfaces of frankfurters and sausages
The U.S. Food and Drug Administration (FDA) has recently announced a notable proposal to remove Orange B color additive from the list of permitted substances.
- Background and Description of the Proposed Order
According to the proposal published on September 17, 2025, in the Federal Register, Orange B was first authorized by the FDA in 1966 for use in coloring the casings of sausages and frankfurters at concentrations not exceeding 150 ppm by weight of the finished product. However, FDA certification records indicate that this additive has not been manufactured or certified for use in the United States since 1978. The fact that Orange B has not been used for nearly half a century demonstrates that the regulation permitting its use has become obsolete and unnecessary.
The removal of this regulation is part of broader directives from the U.S. government, in line with Executive Order 14192 issued by President Trump and initiatives from the Secretary of Health and Human Services (HHS). The proposal to eliminate Orange B also responds to a 2008 petition from the Center for Science in the Public Interest's (CSPI), which called on the FDA to revoke the approval of several color additives, including Orange B. This review process is also consistent with Executive Order 13563 from 2011, which requires federal agencies to regularly reassess existing regulations to amend or repeal them as needed.
- Public Consultation Process
Currently, the public consultation phase is underway and will conclude on October 17, 2025. The FDA is encouraging individuals and organizations to submit comments, particularly through the https://www.regulations.gov electronic portal.
This decision reflects a direction toward streamlining and increasing the flexibility of the U.S. regulatory system, eliminating unnecessary burdens. It may serve as a precedent for a series of similar actions in the future, aimed at modernizing and optimizing the food safety management system in the United States.
- Effective Date
If this proposal is adopted, the regulation repealing Orange B at 21 CFR 74.250 will take effect 45 days after its publication in the Federal Register. After the effective date, any remaining batches of Orange B on the market will no longer be certified, and its use in food will be considered a violation of the law.
Canada
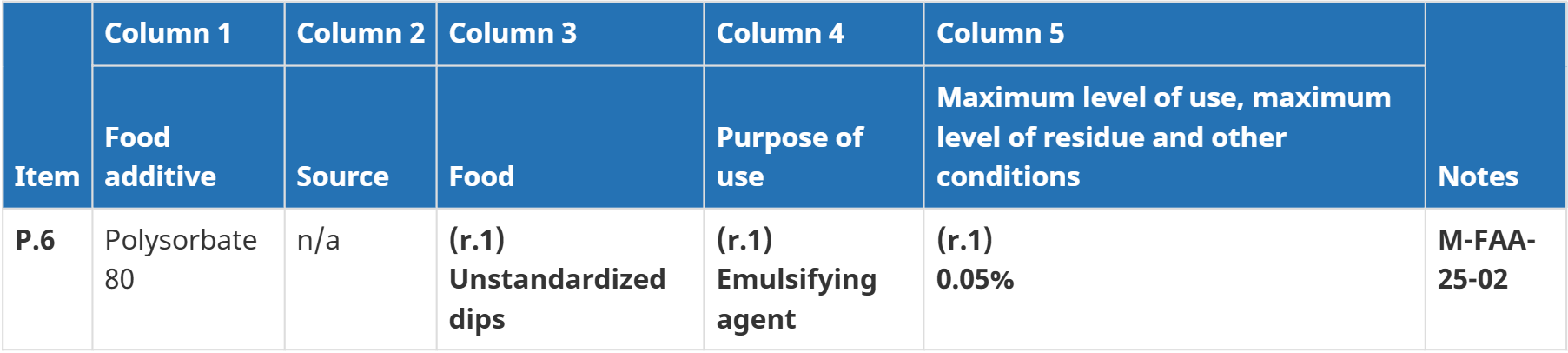
Expands the permitted use of Polysorbate 80 in food products
According to Notice M-FAA-25-02 dated September 15, 2025, Health Canada has officially approved the use of polysorbate 80 as an emulsifier in unstandardized dips at a maximum concentration of 0.05%, effective October 20, 2025. This decision follows a comprehensive safety assessment conducted by the Bureau of Chemical Safety, which confirmed the additive’s safety for this intended use.
- Regulatory Basis
Health Canada’s decision stems from a submitted application requesting the expansion of polysorbate 80’s permitted uses. Although this additive had previously been approved for use in various food categories, it had not been authorized for use in dipping sauces.
To reach a final decision, Health Canada conducted a thorough safety evaluation, covering key aspects such as allergenicity, chemistry, nutrition, and toxicology. The results confirmed that polysorbate 80 is safe for use in this context. Its inclusion will enable manufacturers to produce products with more consistent and appealing textures.
- Amendments to the Official Additive List
To formalize this decision, Health Canada will add polysorbate 80 to the List of Permitted Emulsifying, Gelling, Stabilizing or Thickening Agents, specifically under item P.6. This legal procedure is essential to ensure manufacturers can comply with the regulation lawfully.
In addition to this update, Health Canada is making minor adjustments to restore alphabetical order within the list, including the reordering of sodium stearate and sodium stearoyl-2-lactylate.
- Regulation and Enforcement
Under Canadian law, all food additives must meet strict technical standards. If no specific Canadian standard exists, the additive must comply with internationally recognized specifications such as the Food Chemicals Codex (U.S.) or the Combined Compendium of Food Additive Specifications issued by the FAO/WHO Joint Expert Committee on Food Additives (JECFA).
Once the decision takes effect on October 20, 2025, the Canadian Food Inspection Agency (CFIA) will be responsible for enforcing the new regulations, ensuring that the use of polysorbate 80 aligns with the approved conditions.
This expansion reflects a positive shift, balancing the needs of the food industry with public health protection.
Vietnam
Scientific workshop on Draft national technical regulation QCVN 5-1:2025/BCT on Liquid milk products: Industry proposals and insights
On September 19, 2025, the Ministry of Industry and Trade (MOIT) convened a significant scientific workshop in Ho Chi Minh City to gather feedback on the draft National Technical Regulation QCVN 5-1:2025/BCT concerning liquid milk products. This initiative marks an essential step toward refining the regulatory framework to ensure product quality in the dairy sector, while also providing a constructive platform for enterprises and experts to contribute their proposals.
The workshop served as an effective dialogue forum, bringing together key stakeholders including MOIT leadership, representatives from industry associations, dairy manufacturers, testing laboratories, and experts from research institutes and universities. Under the chairmanship of Deputy Minister Trương Thanh Hoài, the event recorded numerous in-depth contributions focusing on technical, regulatory, and practical feasibility aspects of the draft regulation. Discussions centered on revising certain provisions to better align with the realities of production and business operations.
Key Proposals from the Business Community
1. Scope of Regulation and Product Classification
One of the most discussed topics was the clarification of the regulation’s scope. Delegates proposed amending the phrase “not applicable to health protection food” to “functional food,” in accordance with current legal definitions. The rationale is that functional food is a broader category encompassing health protection food, and clearer terminology would help avoid confusion in product management and declaration. Additionally, businesses advocated for a clear distinction between “reconstituted milk” and “recombined milk” to enhance transparency in product classification, facilitating inspection, supervision, and labeling.
2. Technical Specifications
Regarding milk fat content, many enterprises argued that the current draft does not adequately reflect the diversity of low-fat products available on the market. Given the range of partially and fully skimmed products, businesses proposed a more flexible indicator. Specifically, they suggested merging the categories “partially skimmed pasteurized/UHT milk” and “skimmed pasteurized/UHT milk” into a single group with a unified fat content threshold of <3.2%, rather than maintaining separate limits. This would allow manufacturers greater flexibility in product development to meet modern consumer trends.
Furthermore, concerning the specific gravity of milk at 20°C, delegates pointed out that the proposed minimum of ≥1.026 g/ml for all reconstituted fresh milk products is not appropriate. Specific gravity is a physical indicator reflecting concentration and the natural nutritional composition of milk. While raw milk has a stable specific gravity, products that undergo formulation or contain added ingredients may vary significantly, rendering the indicator less meaningful for assessing quality. Therefore, a reconsideration of this criterion for processed products was recommended.
3. Transition Period Regulations
A particularly emphasized proposal was to extend the transition period from 6 months to 12 months. The justification lies in the time required to reformulate products to meet new standards (such as milk fat content), which involves research, stability testing, and necessary trials. Additionally, sourcing packaging materials from overseas is a time-consuming and costly process. Extending the transition period would provide businesses with more favorable conditions and help avoid unnecessary losses.
4. Transparency in Feedback Collection
To ensure a democratic and effective regulatory development process, MOIT proposed that all feedback from enterprises be documented in writing. This would enable the drafting committee to study, incorporate, and respond to comments based on a solid foundation, ultimately producing a regulation that is both technically rigorous and practically viable.
The workshop successfully created an open forum for stakeholders to collaborate in refining QCVN 5-1:2025/BCT. By actively listening to and incorporating feedback from the business community, the regulation is expected to not only safeguard consumer interests but also foster sustainable growth in Vietnam’s dairy industry.
Japan
Japan's Revised MRLs for Pesticides in 18/09/2025
On September 18, 2025 Japan's Consumer Affairs Agency (CAA) released an amendment to the Maximum Residue Limits (MRLs) for 8 Pesticides and Veterinary drugs : Isofetamid, Dibutylsuccinate, Dimpropyridaz, Thifluzamide, Norgestome, Pyraziflumid, Fluxametamide and Polyoxin complex in certain food products. The amendment will be implemented immediately upon publication. However, certain food products will be implemented one year after publication. The change is as follows:
- MRLs in food have been Immediate revised for the following 7 substances: Isofetamid, Dibutylsuccinate, Thifluzamide, Norgestome, Pyraziflumid, Fluxametamide and Polyoxin complex
- MRLs in food have been newly established for the following substance: Dimpropyridaz.
For example for detail the change implemented immediately in the table below:
|
Active Pesticides |
Food |
After (ppm) |
Before (ppm) |
|
Isofetamid |
Rapeseed oil |
0.03 |
0.01 |
|
Pimento (sweet pepper) |
7 |
0.01 |
|
|
Dimpropyridaz |
Tomato |
1 |
0.01 |
|
Eggplant |
0.6 |
0.01 |
|
|
Apple |
0.4 |
0.01 |
|
|
Grapes |
2 |
0.01 |
|
|
Tea |
30 |
0.01 |
|
|
Honey |
0.05 |
0.01 |
|
|
Pyraziflumid |
Watermelon |
0.9 |
0.8 |
|
Okra |
2 |
0.01 |
|
|
Ginger |
0.06 |
0.01 |
|
|
Persimmon |
2 |
0.8 |
|
|
Fluxamethamide |
Carrot |
0.1 |
0.01 |
|
Spinach |
20 |
0.01 |
|
|
Pumpkin |
0.7 |
0.01 |
|
|
Polyoxin complex |
Honey |
0.05 |
0.01 |
Foods to which residue limits will apply one year after publication:
- Isofetamid: Watermelon (including the peel), Sesame seeds, Rapeseed, Other oil seeds and Other herbs.
- Thifluzamide: Other vegetables.
- Pyraziflumid: Pumpkins (including squash), Melons (including the peel), Japanese gourds (including the peel) and Other cucurbit vegetables
- Fluxametamide: Cabbage, Green onions (including leeks) and Green peppers.
- Polyoxin Complex: Mandarin oranges (including outer peel)
For example for detail the change that will be implemented one year after the date of publication are in the table below:
|
Active Pesticides |
Food |
After (ppm) |
Before (ppm) |
|
Isofetamid |
Watermelon |
1 |
2 |
|
Sesame seeds |
0.01 |
0.02 |
|
|
Rapeseed |
0.01 |
0.02 |
|
|
Thifluzamide |
Other vegetables |
0.01 |
1 |
|
Pyraziflumid |
Pumpkin |
0.9 |
1 |
|
Fluxametamide |
Cabbage |
0.9 |
1 |
|
Polyoxin Complex |
Mandarin oranges |
0.5 |
0.7 |
For details see attached link: https://www.caa.go.jp/policies/policy/standards_evaluation/pesticide_residues/notice/assets/standards_cms208_250918_01.pdf
Thailand
Update Regulations for Bottled Water, Ice, and Beverages
The Ministry of Public Health of Thailand is urgently preparing to issue four new draft notifications aimed at strengthening safety standards for drinking water, ice, natural mineral water, and beverages in sealed containers.
According to documents from the Thai Food and Drug Administration (FDA), these draft notifications are designed to replace and supplement outdated regulations, addressing emerging challenges related to chemical contamination and leveraging modern technological advancements.
Once signed by the Minister of Public Health, the draft laws will be published in the Royal Gazette and officially come into effect. The notifications include:
- Notification No. 462/2568: Drinking Water in Sealed Containers
- Notification No. 463/2568: Ice
- Notification No. 464/2568: Natural Mineral Water (2nd Revision)
- Notification No. 465/2568: Beverages in Sealed Containers (3rd Revision)
These drafts aim to enhance consumer health protection, align with technological progress, and harmonize with international standards. Notable changes include an expanded definition of bottled drinking water, updated contaminant lists, and the introduction of digital labeling as an eco-friendly alternative.
Key Changes
1. Notification No. 462: Drinking Water in Sealed Containers
Replaces Notification No. 61 (1981) and related amendments.
a. Expanded Definition and Scope
The definition of “drinking water in sealed containers” now includes water from identified sources (e.g., spring water) and carbonated water (soda water), aligning with international standards.
b. Stricter Microbiological and Chemical Standards
The draft updates contaminant limits based on WHO recommendations and Codex Alimentarius standards. A total of 44 chemical contaminants are regulated, grouped into four categories:
- Inorganic substances (16 types): Includes lead, arsenic, mercury, chromium—naturally occurring from geological and climatic factors, including mineral accumulation in eutrophic water bodies.
- Organic chemicals and VOCs (14 types): Includes benzene, toluene, vinyl chloride—originating from industrial and domestic activities.
- Pesticides (7 types): Includes atrazine, carbofuran, DDT—targeting agricultural pollution.
- Disinfection by-products (7 types): Includes bromate, chlorite—resulting from water treatment processes.
Contaminant testing is tailored to water source characteristics:
- Forest reserves or Type 1 surface water: Test only inorganic substances.
- Urban, commercial, industrial areas (Type 4, 5 surface water): Test inorganic and organic substances.
- Rural and agricultural areas (Type 2, 3 surface water): Test inorganic substances and pesticides.
- Sources using disinfectants: Must also test for disinfection by-products.
Microbiological standards:
- Coliforms and E. coli: New limit is <1.1/100ml (MPN method).
- Pathogens: No detection of Salmonella in 100ml; Staphylococcus aureus must be <100 CFU/100ml.
c. Digital Labeling Implementation
Manufacturers may opt out of traditional plastic labels and ink printing. Instead:
- Basic product info (name, registration number, volume, production/expiry date) must be embossed or engraved directly on the bottle.
- A QR code must be displayed on the cap or bottle body, linking to a webpage with full product details—reducing plastic waste.
2. Notification No. 463: Ice
Replaces Notification No. 78 (1984)
- Standard Alignment: Water used for ice production must meet all chemical and microbiological standards outlined in Notification 462.
- Production Requirements: Detailed production methods are replaced with general Good Manufacturing Practices (GMPs).
- Mandatory Labeling: All ice products must clearly state “Ice for consumption” on packaging.
3. Notification No. 464: Natural Mineral Water
Focuses on labeling revisions
Digital Labeling: Similar to bottled drinking water, producers may use digital labels to reduce plastic waste and ink-related contamination risks.
4. Notification No. 465: Beverages in Sealed Containers
Revises beverage classification for international harmonization
- Reclassification: Beverage categories reduced from five to four.
- Reassignment: Products containing added carbon dioxide or oxygen are reclassified under bottled drinking water (Notification 462), ensuring consistency.
- International Alignment: Changes comply with Codex GSFA food categorization, facilitating global trade and regulatory coherence.
5. Transition Periods
- Drinking Water and Beverages: Manufacturers have 2 years to comply with Notifications 462 and 465.
- Ice: Ice producers have 1 year to adapt to Notification 463.
All notifications will take effect the day after their publication in the Royal Gazette.
Link: https://food.fda.moph.go.th/press-release/enforce-4water
|
This is an electronic newsletter updating regulations and laws on food hygiene and safety in domestic and international markets; Compiled by the Advisory Department of Eurofins Sac Ky Hai Dang based on government official pages of the countries, published every month. We encourage you to use this as a reference channel for information and exemption from liability related to making business decisions at your company or other similar activities. For detailed inquiries, please contact:
|
Read more related news
- Update on the latest changes and regulations in the EU market
- How worried should you be about Aspartame?
- Update valid regulations from 2023
- Sesame allergen labeling required in the US market
- Update on food nutrition labeling regulations 2022
- Latest updates on Ethylene Oxide regulations in the EU market
- Mineral oil (MOSH/MOAH) contamination in Food
- Analysis Furan and Alkylfurans residues in food

To send samples or find more information, please contact us at:
Eurofins Sac Ky Hai Dang
Hotline: (+84) 28 7107 78 79 - press 1 (Sales Dept.)
Email: VN_CS@eurofinsasia.com

